
III. Cell Potentials Under Standard Conditions B. Silver - Copper Cu(s), CuCl2 (1.0 M) || Ag(s), AgNO3 (1.0 M) 1. Draw the schematic of the electrochemical cell that includes all the components (

Solve this: 8 A Copper-Silver cell is set up The copper ion - Chemistry - Electrochemistry - 12485096 | Meritnation.com
A copper_ silver cell is set up. The copper ion concentration is 0.10 M. The concentration of silver ion is not know.the cell potential when measured was 0.422V determine the concentration of

13.A copper-silver cell is up. The copperion concentration is 0.10M. The concentration of Silver ions is not known. The cell potential was found to be 0.422V.Determine the concentration Silver ion in

A copper - silver cell is set up. The copper ion concentrations is 0.10 M. The concentration of... - YouTube

13.A copper-silver cell is up. The copperion concentration is 0.10M. The concentration of Silver ions is not known. The cell potential was found to be 0.422V.Determine the concentration Silver ion in
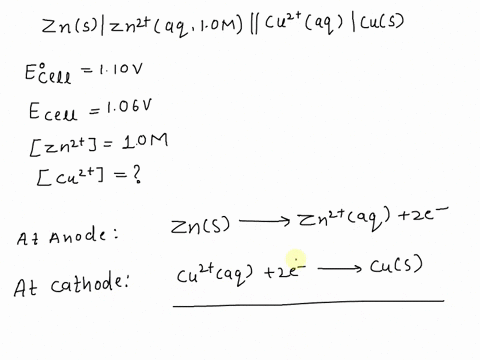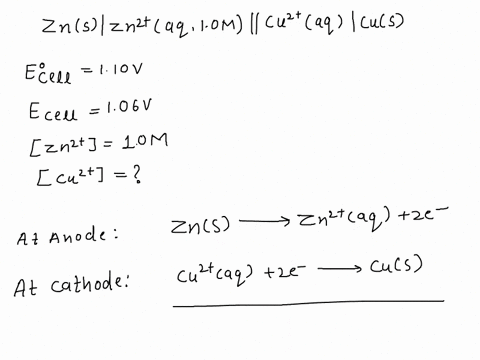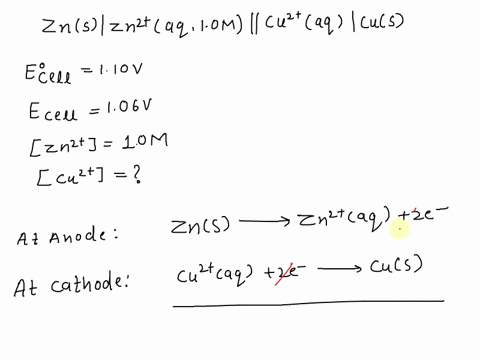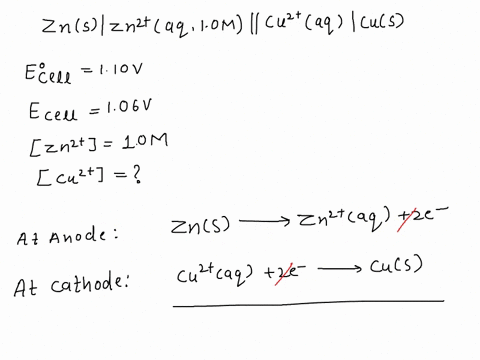
SOLVED: A copper-silver cell is set up. The copper ion concentration in it is 0.10 M. The concentration of silver ion is not known. The cell potential is measured as 0.422 V.

A copper- silver cell is set up. The copper ion concentration in it is 0.10 M. The concentration of silver ion is not known. The cell potential measured 0.422 V. Determine the

SOLVED: The Cu2+ ion concentration in a copper-silver electrochemical cell is 0.5 M. If Eo (Ag+/Ag) = 0.9 V, Eo (Cu2+/Cu) = 0.34 V, and cell potential (at 25°C) = 0.422 V,
BIM objects - Free download! Copper-Silver Ionization System - CSI - 2 Flow Cell 2 Controller Rack Mount - 2F2C | BIMobject
SOLVED: The Cu2+ ion concentration in a copper-silver electrochemical cell is 0.5 M. If Eo (Ag+/Ag) = 0.9 V, Eo (Cu2+/Cu) = 0.34 V, and cell potential (at 25°C) = 0.422 V,

HULUSUL PURCULUI 13.A Copper-silver cell is up. The copper ion concentration in it is 0.10M.The concentration of silver is not known. The cell potential measured is 0.422V.Determine the concentration of silver ion

7. of A copper silver cell is setup. The copper ion concentration in it is 0.10 M. The concentration of silver ion is not known. The cell potential measures 0.422V. Determine the
A copper-silver cell is set up. The copper ion concentration in it is 0.10 M. The concentration of silver ion is not known. - Sarthaks eConnect | Largest Online Education Community






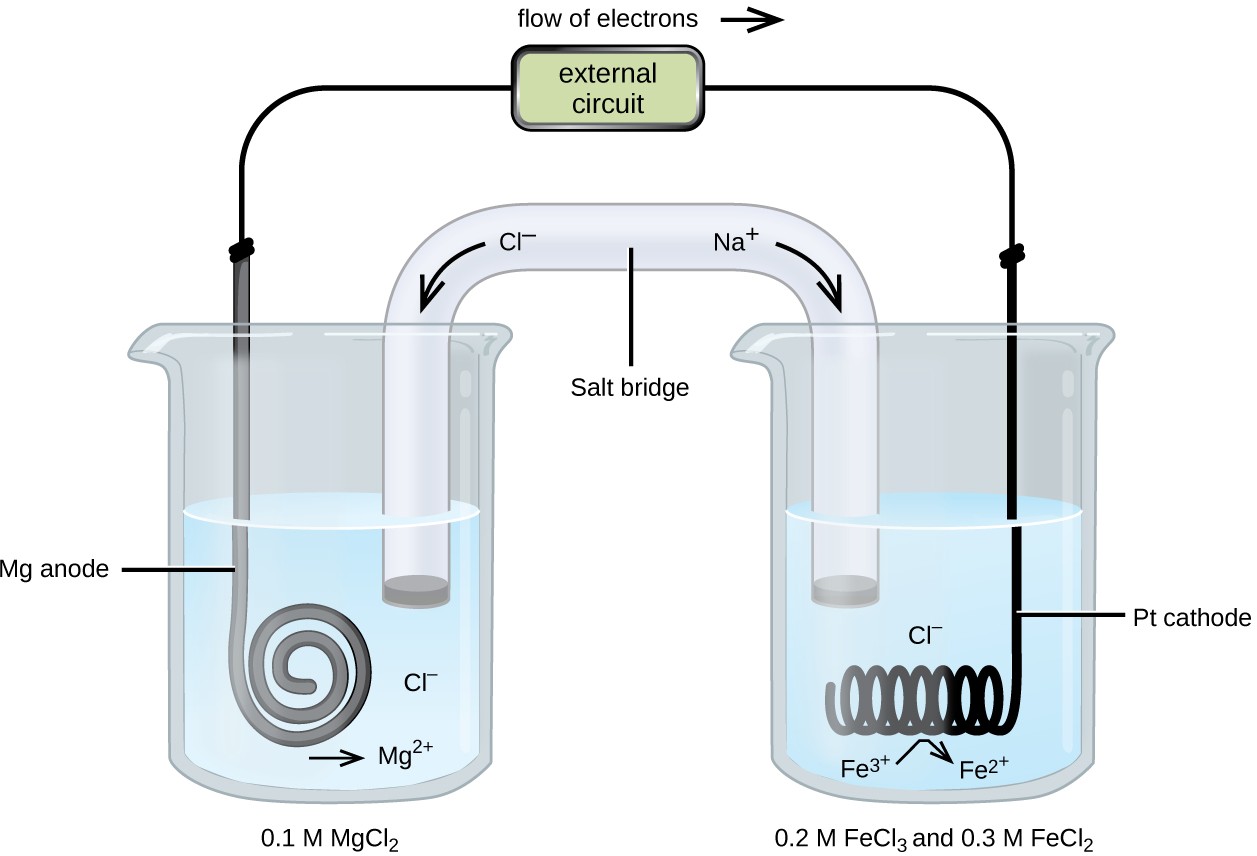
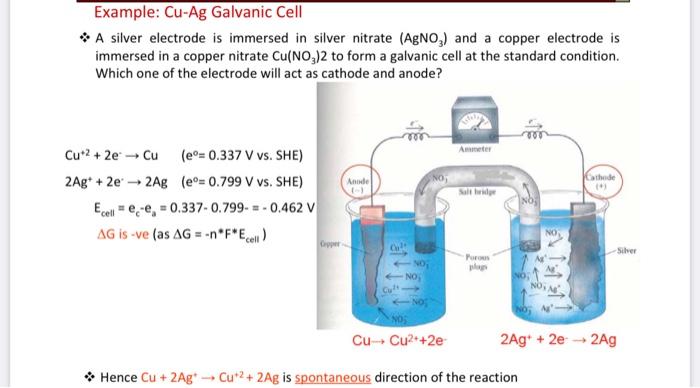


![Assamese] A copper silver cell is set up. The copper ion concentrati Assamese] A copper silver cell is set up. The copper ion concentrati](https://static.doubtnut.com/ss/web-overlay-thumb/8484396.webp)